Our Management is a true combination of leadership, talent, scientific knowledge, and ability to work as a team. An interdisciplinary group of people with a history of success, enriched over time by newcomers with a proven track record who have fuelled the Company with enthusiasm and experience.
Lucio Rovati, MD
Chief Executive Officer – Chief Scientific Officer
 Lucio Rovati joined the Company in 1988, following the footsteps of his father, Professor Luigi Rovati who was the founder of the Rottapharm Group.
Lucio Rovati joined the Company in 1988, following the footsteps of his father, Professor Luigi Rovati who was the founder of the Rottapharm Group.
After graduation in Medicine and Postgraduate specialization in Clinical Pharmacology at the University of Milan, he completed his training as attending physician in Internal Medicine and served as research fellow in the Department of Medical Pharmacology, Chemotherapy and Toxicology of the University of Milan, School of Medicine, for four years.
In Rottapharm, he served as head of Clinical Pharmacology and Clinical Research before becoming Chief Scientific Officer, directing all R&D activities of the Group, and Executive Medical Director responsible for all international medical activities of the Company and its subsidiaries. He founded Rottapharm Biotech in 2014 and currently is Chief Executive Officer and Chief Scientific Officer of the Company.
Besides his experience in internal medicine, Lucio has laboratory skills on in vitro and in vivo pharmacology, particularly of the musculoskeletal, digestive and central nervous systems. However, his most relevant expertise derives from his wide clinical research activity in the direction (including planning, performance and reporting) of clinical research programs and studies for the development of new drugs in rheumatology, gastroenterology/hepatology, gynaecology, bronchopneumology, neurology/psychiatry, cardiology, metabolism and internal medicine.
After holding similar positions at the Universities of Naples and of Parma, Dr Rovati is at present and since 2003 Invited Professor of Clinical Pharmacology at the University of Milano-Bicocca, School of Medicine, where he is also Coordinator of a Postgraduate Master in Pharmaceutical Medicine.
Dr Rovati is an active member of several international scientific societies in different disciplines, where he is also included in panels providing advice to the Food and Drug Administration or the European Medicine Agency on the drafting of drug development guidelines.
Finally, he is the author of over 175 full papers in peer reviewed international scientific journals (H-index=42), 15 book chapters and over 400 abstracts. Dr Rovati has been among the ten Italian scientists with the largest number of publications in clinical research.
Federica Girolami, PharmD, MSc
Business Development, Scientific Liaison and Drug Safety – Director
Federica Girolami joined the Company in 2006 and currently serves as Business Development, Scientific Liaison and Drug Safety Director. In Rottapharm Biotech she provides strategic support to the Chief Executive Officer in negotiations and strategic alliances networking to attract external partners. She is responsible for in-licensing, out-licensing and partnering activities, and for identifying opportunities for Rottapharm Biotech’s assets across all therapeutic areas. In her role of Scientific Liaison, she is responsible for cultivating and maintaining relationships with academic researchers and key opinion leaders, acting as a bridge of communication for both external and internal stakeholders.
Additionally, given her long-standing experience in Pharmacovigilance and Drug Safety, she is responsible for all Drug Safety activities in Rottapharm Biotech’s Clinical Trials.
Federica Girolami holds a Pharmacy degree from the University of Florence and a Master degree in International Health Care Management, Economies and Policy from the SDA Bocconi School of Management in Milan. Prior to joining Rottapharm, she had gained significant experience in the international arena, with roles of increasing responsibility in multinational pharmaceutical companies in Europe. After starting her career at Du Pont Pharma in Florence, she joined the Swiss headquarters of Helsinn Healthcare, where she contributed to one of the first EMA safety referrals and authored the first pharmacoeconomic publication of a new antiemetic drug in oncology. Very early in her career, she took the responsibility of creating the company Pharmacovigilance and Drug Safety function at IBSA, eventually becoming European Qualified Person responsible for Pharmacovigilance (QPPV) for the group.
In the former Rottapharm|Madaus Group she served as Director Corporate Pharmacovigilance and Drug Safety Department and Deputy QPPV for 8 years coordinating the function globally. In 2015, she was advanced to her current role to provide strategic support to Rottapharm Biotech’s Chief Executive Officer.
Nadia Brambilla, MSc
Clinical Operations – Director
Nadia Brambilla joined the Company in 2006 and currently serves as Clinical Operations Director.
At Rottapharm Biotech she is responsible for coordinating and managing all aspects of the company clinical trials. She also supports the CEO/CSO in the evaluation of possible investments and in- and out-licensing projects.
Nadia holds a master degree in Biological Science from the University of Milan, a 2-year post degree specialization in Pharmacology (University of Milan) and a 3-year post degree specialization in Regulatory Affairs (University of Pavia).
Prior to joining Rottapharm, she spent more than 10 years working in preclinical research at Zambon Group pharmacology laboratories, and 7 years in clinical research, in different CROs, working as project manager and as clinical team leader.
Giampaolo Giacovelli, PhD
Biostatistics – Director
Giampaolo Giacovelli joined the Company in 1987 and currently serves in Rottapharm Biotech as Director, Biostatistics, with responsibility on biostatistics, data management and bioinformatics. He also supports the CEO/CSO in the evaluation of possible investments and in- and out-licensing projects.
Giampaolo Giacovelli holds a degree in Biological Sciences from the University of Milan and a Diploma in Medical Physics and Radioisotopic Techniques from the Politecnico University of Milan. In nearly 40 years of career in the pharmaceutical industry, he held different positions of rising responsibility and matured a wide experience in drug development, mainly in the clinical phases. Thanks to a deep involvement in the design and conduct of multiple preclinical/clinical international projects, he has gained extensive expertise in several therapeutic areas such as gastroenterology, gynaecology, bronchopneumology, hepatology, and rheumatology.
His expertise includes, among others, the development of innovative drugs in the fields of irritable bowel syndrome (IBS), hormone replacement therapy, contraception, asthma, hepatitis C, analgesia, osteoporosis and especially osteoarthritis. Indeed, Giampaolo has gained an international reputation as a clinical trial methodologist in osteoarthritis.
Marta Monteforte, BMath
Biostatistician and Data Management Specialist
Marta Monteforte joined the Company in 2024 and currently serves as Biostatistician and Data Management Specialist.
At Rottapharm Biotech she performs statistical analyses of clinical studies in different indications, writes the statistical section of the study protocol and of the clinical study report. In her role, Marta supervises outsourced data management activities. Furthermore, she supports the CEO/CSO in the evaluation of possible investments in-licensing and out-licensing projects.
Marta holds a Statistical, Demographic and Social Sciences degree from the Bicocca University of Milan, a master’s degree in Biostatistics and Experimental Statistics (Bicocca University of Milan) and a Master on Biomedical Research (Mario Negri Institute of Pharmacological Research, Milan).
Prior to joining Rottapharm, she spent more than 7 years working as a Researcher and Statistician at Mario Negri Institute of Pharmacological Research in Milan and 5 years in clinical research, in different CROs, working as data manager and statistician.
Francesca Fanti, PharmD
Regulatory and Quality Affairs – Director
Francesca Fanti joined the Company in 2010 and currently serves as Regulatory and Quality Affairs Director. At Rottapharm Biotech she is responsible for Regulatory Affairs activities and strategy within the Company, in order to expedite approval of regulatory applications supporting clinical trials and to address specific questions from Regulatory Authorities. She is also responsible for managing and coordinating Quality Control outsourced activities (e.g. stability studies, analytical methods set up and validation, development investigations) through liaison with technical functions, including consultants/vendors qualification, and appropriate Standard Operating Procedures set up. She also supports the CEO/CSO in the evaluation of possible investments and in- and out-licensing projects.
Francesca Fanti holds a Pharmaceutical Chemistry and Technology degree from the University of Bologna. Prior to joining Rottapharm, she spent almost 20 years at GlaxoSmithKline where she developed her career and gained a deep understanding of the pharmaceutical and biotechnology industry. Initially she was in charge of quality aspects and organization of the Departments in terms of GMP, GLP, Standard Operating Procedures (SOPs), training, and audits. In 2001 she became part of the Global Regulatory Affairs CMC (Chemistry, Manufacturing and Controls) Pre-Approval Department based in UK/US. In this role she coordinated, prepared and delivered the CMC components of global regulatory applications for new chemical entities and product line extensions from First-in-Humans studies to global marketing approval, including regulatory strategy for new drug substances and drug products delivery and supply, and led Global Regulatory Affairs teams based in Europe and North America.
Michela Visintin, PhD
Biotherapeutics Discovery and Development – Director
Michela Visintin joined the Company in 2008 and currently serves as Biotherapeutics Discovery and Development Director. She also supports the CEO/CSO in the evaluation of possible investments and in- and out-licensing projects.
Michela holds a Science Biology degree from the University of Trieste and a PhD in Biophysics from the International School for Advanced Studies in Trieste.
Prior to joining Rottapharm Biotech, she was head of the Antibody Technology Unit at Lay Line Genomics, a spin-off of the International School for Advanced Studies (SISSA) with the mission to discover and develop innovative biopharmaceuticals for the treatment of neurological diseases. During her PhD and later on in Lay Line Genomics, she worked on selection methods for isolating intracellular antibodies (intrabodies) and related methods for disease target validation and discovery of innovative biotherapeutics. All these technologies have been industrially exploited in Rottapharm Biotech. She also co-authored several scientific papers and patents in the field of biotechnology and protein knock-out technology.
In Rottapharm Biotech she is responsible for the discovery of novel biotherapeutics. Thanks to over 20 years of experience in biotechnology development and protein engineering, Michela played a key role in establishing the first Rottapharm Biotech’s antibody lead discovery capability.
Tiziana Piepoli, PhD
Pharmacology Scientist
Tiziana Piepoli joined the Company in 2002 and currently serves as Pharmacology Scientist. Tiziana Piepoli holds a degree in Pharmaceutical Chemistry and Technology from the University of Milan, where she also completed her post-doctoral training, and specialized in Pharmacology.
In Rottapharm Biotech she is responsible for the design, conduct and analysis of pharmacology and toxicology studies. She also supports the CEO/CSO in the evaluation of possible investments and in- and out-licensing projects.
Tiziana started her career in Germany working in preclinical research at the University J. Gutenberg, Mainz, where she also trained and evaluated students in Biochemistry. Then she moved to the Hospital C. Besta in Milan. She acquired also 2 years of experience in the clinical research at the hospitals Sacco and Fatebenefratelli in Milan. She is co-author of several papers, a chapter of a book on brain tumours and different patents.
Luigi Giancotti, PhD
Pharmacology Scientist
Luigi Giancotti joined the Company in 2023 and currently serves as a Pharmacology Scientist. In Rottapharm Biotech he is responsible for designing, conducting, analyzing and monitoring pharmacological and toxicological studies. He also supports the CEO/CSO in the evaluation of possible investments and in- and out-licensing projects.
Luigi holds a Bachelor of Science in Biology (University of Messina), a Specialist Degree in Biology applied to Biomedical Research (University of Roma Tre) and a PhD in Pharmacology (Sapienza, University of Rome). He started his career as a Researcher in the laboratory of IRCCS San Raffaele Pisana (Rome) as Post-Doc Fellow and then moved to Saint Louis University (St. Louis, USA) where he worked as an Assistant Research Professor in the Department of Pharmacology and Physiology before joining Rottapharm Biotech. During his academic career he led several projects aiming to understand the molecular and pharmacological basis of acute and chronic pain states. He trained, mentored and guided more than 10 students and he contributed to the publication of more than 20 papers on peer-reviewed journals.
Gianfranco Caselli, PhD
Pharmacology and Toxicology – Advisor
Gianfranco Caselli joined the Company in 2001 as Pharmacology and Toxicology Director, and after retirement he continues to support Rottapharm Biotech as an Advisor. Gianfranco also supports the CEO/CSO in the evaluation of possible investments and in-licensing projects.
Gianfranco Caselli holds a degree in Pharmaceutical Chemistry and Technology from the University of Milan, where he also completed his post-doctoral training in Pharmacognosy at the Institute of Pharmacology, School of Pharmacy, and specialized in Toxicology. Gianfranco started his career as Manager of the Laboratory of Nutritional Biochemistry at Pierrel, and then moved to Dompé where he worked for fifteen years, covering different management roles up to the direction of Pharmacology and Toxicology.
Roberto Artusi, MChem
Medicinal Chemistry Discovery and Development – Director
Roberto Artusi holds a degree in Chemistry from the University of Milan. He joined the Company in 1997 as a synthetic chemist and, since then, has been approaching various aspects of medicinal chemistry and project management. Through the years, Roberto has played a pivotal role in developing and implementing the processes that now, at Rottapharm Biotech, are used to streamline the transition of original molecules from discovery to preclinical development. Roberto is also taking care of the GMP production of small molecules for clinical study.
He has been involved in many projects on different areas, particularly in rheumatic diseases (osteoarthritis, rheumatoid arthritis) and CNS diseases (sleep disorders, drug addiction).
Roberto currently serves as Medicinal Chemistry Discovery and Development Director and also supports the CEO/CSO in the evaluation of possible investments and in- and out-licensing projects and in the management of the Company’s IP portfolio.
Matteo Ghirri, PharmD
Pharmaceutical Development – Director
Matteo Ghirri joined the Company in 1998 and currently serves as Pharmaceutical Development Director. He also supports the CEO/CSO in the evaluation of possible investments and in- and out-licensing projects.
Matteo holds a degree in Pharmaceutical Chemistry and Technology from the University of Milan. Prior to joining Rottapharm he gained significant experience in standard and controlled-/modified-release formulation methodologies, as well as in process development. At Rottapharm he extended his expertise to the development of a wide range of pharmaceutical products including transdermal delivery systems, injectable products, and medical devices. He supported many industrial operations of scale-up or manufacturing site relocation of pharmaceutical as well as dermo-cosmetic and nutraceutical products. He also acquired the expertise for the development of drug products for clinical trials. In 2005 he was responsible of the activation of the Rottapharm’s Investigational Medicinal Products packaging unit.
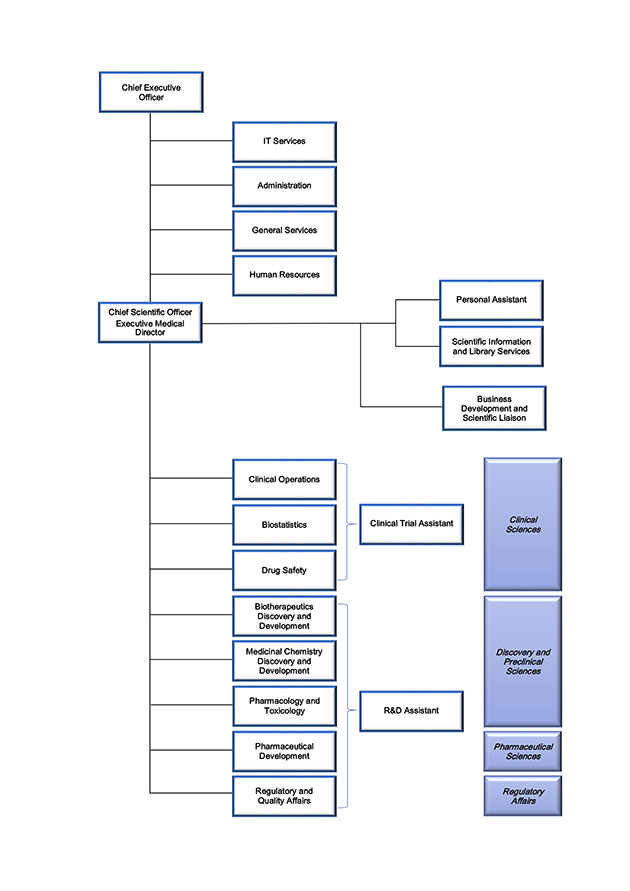
Organizational Chart